Discovery of electron
Discovery of electron :
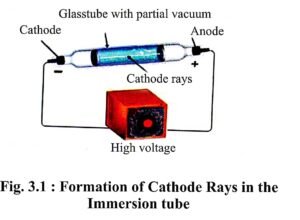
- J.J. Thomson created high vacuum in immersion tube and applied high voltage on the metal electrode. He observed that green fluorescence is generated on the walls of the tube.
- With the help of his experiments, Thomson confirmed that on applying high voltage in vacuum there is flow of electricity in the tube from cathode to anode, in the form of rays. Thomson termed these rays – the cathode rays.
Properties of Cathode rays :
- They move in straight line.
- They produce fluorescence.
- They are affected by electric and magnetic fields.
- When they pass through a gas, they ionise it.
- They are made up of negatively charged particles.
- The e/m (charge/mass) i.e. ratio of charge to mass of these particles is uniform.
- Primarily they are the matter waves.
- According to J.J. Thomson, cathode rays are made up of negatively charged particles which he named as the electrons and their mass was found to be 1/1837 of the hydrogen atom.
- Experiments on the properties of the cathode rays proved that in the atom there is a negatively charged particle, electron, which can be separated from the atom. The atomic electricity is neutral, hence the remaining part of the atom from which electron have been removed, should be positively charged.
Important Points :-
- The basic particles of atom are electron, proton and neutron.
- The negatively charged particles in the atom are electrons.
- The numeric value of the charge on electron and proton is the same but their sign is opposite.
- James Chadwick discovered neutrons.
- There are 6.022×1023 particles in one mole. This is known as the Avogadro number.
- The NTP volume of 1 mole of a gas is 22.4 litres.
- The formula to determine the maximum number of electrons in a shell is 2n2 .
- When the atomic number is the same but mass number is different they are known as Isotopes.
- Isobars are elements having different atomic number and the same mass number.
- There are three isotopes of hydrogen, Protium, Deutirium and Tritium.
Atomic Structure Important Questions-Answers
1. The Plum Pudding Model of atom was given by:
(a) Neil Bohr
(b) Thomson
(c) Ernest Rutherford
(d) Goldstein
Click to show/hide
Answer⇒ { B}
2. The discoverer of neutron was :
(a) C.V. Raman
(b) Rutherford
(c) J.J. Thomson
(d) James Chadwick
Click to show/hide
Answer ⇒ { D}
3. The size of atom is :
(a) 10-6 cm
(b) 10-15 cm
(c) 10-2 cm
(d) 10-8 cm
Click to show/hide
Answer ⇒ {D }
4. The number of neutrons in the Deutirium Isotope of hydrogen is /are :
(a) one
(b) Two
(c) Three
(d) Not even one
Click to show/hide
Answer ⇒ {A }
