What is Matter | Definition, Characteristics, Types, States, Examples, & Facts
What is Matter
- Different objects present around us, likewater, air, salt, book, computer etc., are all matter.
- Every object that occupies space has mass and which can be sensed with the five sense organs, is termed as matter.
- When we say that matter has mass, it means that it has weight. The more heavy an object is, greater will be its mass. Matter occupies space means that it has volume.
Properties of Matter :
- There is empty space between the particles of matter.
- The particles of matter are in a state of constant motion.
- The particles of matter attract each other.
Types of Matter
- Matter can be divided into two types on the basis of its components :
- Pure Matter : Matter which has only one type of ingredient or component is known as pure matter.
For example : Iron, Gold, Water, Oxygen, etc. Elements and components are pure
matter. - Impure Matter : Matter which has more than one type of ingredients or components are known as impure matter.
For example : Cold drink, soil, air etc. Mixtures are impure matter.
States of Matter :
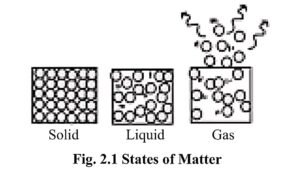
- On the basis of physical state, matter can be classified into three phases :- (i) Solid (ii) Liquid (iii) Gas
For example : - H2 O(g) gaseous phase – steam
- H O(g) liquid phase – water 2 (l)
- H2 O(g) solid phase – Ice
Now scientists are considering five states of matter by including
(iv) Plasma
(v) Bose – Einstein Condensate (BEC)
Characteristic Properties of Matter:
The three basic states of matter can be identified on the basis of their specific properties.
1. Solid State:
- There are numerous substances in solid form all around us. For example : Piece of wood, stone, pencil, pen, computer, salt etc. Following are the characteristic properties of the solid state :
- (i) Solid has a definite shape
(ii) Solid has a definite volume
(iii) The density of solid is more
(iv) Compressibility of solid is negligible
(v)Ahigh inter-molecular force of attraction is present in between the particles of solid state.
(vi) Diffusion in particles of solid is extremely less.
2. Liquid State :
- Water, mustard oil, kerosene etc. are the examples of liquid. The volume of liquid is definite but its shape is not; they take the shape according to the vessel. Liquid can flow. Liquid can be poured or spread. The properties of liquid are intermediate between solid and gas.
- The characteristic properties of liquid state are :
- (i) Shape of liquid is not definite.
(ii) Volume of liquid is definite.
(iii) The density of liquid is more than that of gas but less than that of solid.
(iv) The compressibility of liquid is very less.
(v) The inter-molecular force of attraction between the particles of liquid is weak.
(vi) Diffusion in particles of liquid is less than that in gas but more than that in solid.
3. Gas State :
- The air present around us is the best example of the gas state, other examples include – Oxygen,
Nitrogen, Argon, Carbon-di-oxide etc. - Following are the characteristic properties of the Gas state :
(i) The shape of gas is not definite and it takes the shape of the vessel, in which it is placed.
(ii) The volume of gas is not definite and it take its volume according to the shape of the vessel.
(iii) The density of gas is very less.
(iv) The compressibility of gas is very high.
(v) The inter-molecular force of attraction between the particles of gas is negligible.
(vi) The diffusion in the particles of gas is very high. Hence it quickly spreads every where.
The distance between the particles of gas is too much. They can be brought near each other by applying high pressure and reducing the temperature and can be liquefied. The name of CNG, used as a fuel, is Compressed Natural Gas. LPG is Liquefied Petroleum Gas.
Facts of Matter :-
- 1. Water, Air, Stone – all are matter.
- 2. There are primarily three physical states of matter – solid, liquid and gas.
- 3. The information regarding atom was first of all given by Maharshi Kanad.
- 4. Atomism was given by John Dalton.
- 5. Molecules are formed by chemical combination of atoms in a definite proportion.
- 6. Matter is present in the form of element, compound and mixture.
- 7. There are two type of molecules – elemental and compounds.
- 8. Mixture is formed by combining matter in indeterminate or definite proportions.
- 9. Conversion of ice into water is a physical change while breaking of ice molecules into hydrogen (H2 ) and oxygen (O2 ) is a chemical change.
- 10. The matter can be purified by crystallisation, distillation, fractional distillation, differential extraction, filteration etc.
