Atomic weight | Definition, Units, & Table
What is Atomic weight :-
- With the studies regarding atomic structure, the knowledge of e/m (i.e. ratio of charge to mass) of the electron had developed. Over period of time, even the atomic weight was determined. In the beginning the weight of atoms was calculated in relation to that of the smallest atom, i.e. of hydrogen, which was taken as unit. The atomic weight was defined as – “The atomic weight of an element is that number which indicates how heavy is the atom of the element as compared to the hydrogen atom”.
- In 1961, the twelfth part of Carbon-12 isotope was accepted as the international standard atomic mass unit. According to this “the atomic weight of any element is the average weight of all the isotopes of that element as compared to one twelfth part of the carbon-12 isotope”. In other words it is the ratio of the average mass of all the isotopes of that element to one twelfth of the mass of an atom of carbon-12.
- Atomic weight of an element = Weight of one atom of the element / Weight of 1/12 part of the C-12 isotope.
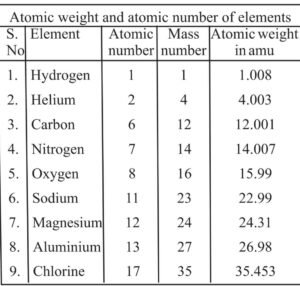
Important Points :-
- The basic particles of atom are electron, proton and neutron.
- The negatively charged particles in the atom are electrons.
- The numeric value of the charge on electron and proton is the same but their sign is opposite.
- James Chadwick discovered neutrons.
- There are 6.022×1023 particles in one mole. This is known as the Avogadro number.
- The NTP volume of 1 mole of a gas is 22.4 litres.
- The formula to determine the maximum number of electrons in a shell is 2n2 .
- When the atomic number is the same but mass number is different they are known as Isotopes.
- Isobars are elements having different atomic number and the same mass number.
- There are three isotopes of hydrogen, Protium, Deutirium and Tritium.
Atomic Structure Important Questions-Answers
1. The Plum Pudding Model of atom was given by:
(a) Neil Bohr
(b) Thomson
(c) Ernest Rutherford
(d) Goldstein
Click to show/hide
Answer⇒ { B}
2. The discoverer of neutron was :
(a) C.V. Raman
(b) Rutherford
(c) J.J. Thomson
(d) James Chadwick
Click to show/hide
Answer ⇒ { D}
3. The size of atom is :
(a) 10-6 cm
(b) 10-15 cm
(c) 10-2 cm
(d) 10-8 cm
Click to show/hide
Answer ⇒ {D }
4. The number of neutrons in the Deutirium Isotope of hydrogen is /are :
(a) one
(b) Two
(c) Three
(d) Not even one
Click to show/hide
Answer ⇒ {A }
