Purification of Matter or Organic Compounds
Purification of Matter :
- In nature majority of the matter is present in impure form. Hence their purification is essential. There are different methods of purifying different substances.
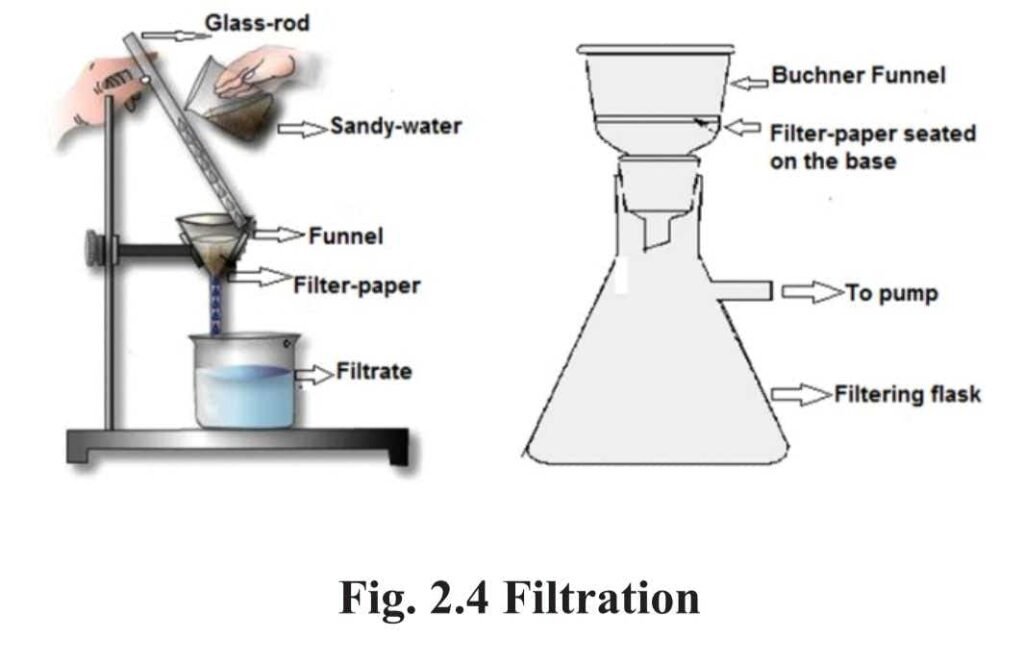
1. Filtration :
- Filtration is a method to separate solid from liquid in a heterogenous mixture. In filtration the solid matter is collected on the filter paper in the form of a residue and the liquid is obtained as filterate, when the mixture is passed through a filter paper. Example : Separating water from the sandy water .
2. Crystallisation :
- Crystallisation is the phenomenon of formation of solid crystals from the saturated solution.
- The crystallisation technique of separating solid from liquid starts with vapourisation. However, in crystallisation when the solution becomes very concentrated, vapourisation is stopped. The concentrated solution thus obtained when cooled gradually, results in the formation of crystals, which can be seperated by filteration. For example – seperating sugar from sugar syrup, making crystal sugar, obtaining salt crystals from saline solution etc.
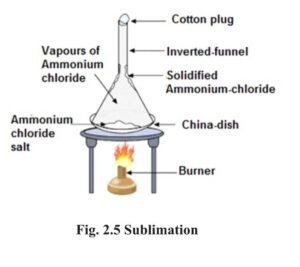
3 Sublimation :
- Sublimation is the property of some substances, by virtue of which, they change directly from solid state to vapour state without liquifying and the vapours when cooled form solid without changing over to the liquid state. For example : Ammonium chloride (Sal ammoniac), iodine, camphor, Naphthalene etc exhibits the property of sublimation.
- Separating a mixture of salt and sal ammoniac (Sodium chloride and Ammonium chloride) :
- On being heated, the sal ammoniac from the mixture vapourises while the salt is left behind. These vapours cool down on the inverted funnel placed on the mixture being heated and thus converts back into pure sal ammoniac.
4 Differential extraction :
- This is a technique to separate two immiscible liquids from each other. For example oil and water.
- When poured in a separating funnel the two liquids in the mixture form separate layers. When the stopcork is opened, the heavy liquid comes out first then the lighter liquid is obtained.

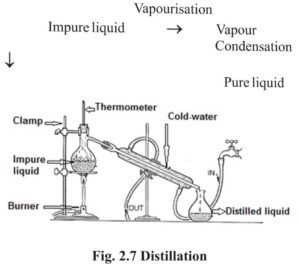
5 Distillation :
- When soluble solid is present in a liquid, the liquid from the mixture vapourises on being heated and on cooling these vapours, pure liquid is obtained because of condensation. This process is known as distillation. Example water distillation etc.
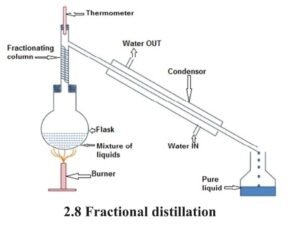
6 Fractional Distillation :
- If the boiling point of two liquids do not have sufficient difference, they cannot be separated by simple distillation. Such liquids vapourise at the same temperature range and then condense together. In such a situation fractional distillation is made use of.
- When the mixture is heated each liquid vapourises at its boiling point. Their vapours when passed through fractioning column and condensed, yield the different liquids. First the liquid with lower boiling point is obtained and then, that with higher boiling point. Example : Various components like petrol, diesel, kerosene, vaseline etc are separated from petroleum by fractional distillation.
Important Points :-
- 1. Water, Air, Stone – all are matter.
- 2. There are primarily three physical states of matter – solid, liquid and gas.
- 3. The information regarding atom was first of all given by Maharshi Kanad.
- 4. Atomism was given by John Dalton.
- 5. Molecules are formed by chemical combination of atoms in a definite proportion.
- 6. Matter is present in the form of element, compound and mixture.
- 7. There are two type of molecules – elemental and compounds.
- 8. Mixture is formed by combining matter in indeterminate or definite proportions.
- 9. Conversion of ice into water is a physical change while breaking of ice molecules into hydrogen (H2 ) and oxygen (O2 ) is a chemical change.
- 10. The matter can be purified by crystallisation, distillation, fractional distillation, differential extraction, filteration etc.
Important Questions-Answers
Q. 1 When did Maharshi Kanad provided information regarding atom?
(a) 500 BC
(b) 100 BC
(c) 460 BC
(d) 1808 AD
Click to show/hide
Q. 2 The atomicity of oxygen in ozone is :
(a) 1
(b) 2
(c) 3
(d) 4
Click to show/hide
Q. 3 Which of the following substance is not solid at room temperature ?
(a) Salt
(b) Alum
(c) Oxygen
(d) Sal ammoniac
Click to show/hide
Q. 4 The temperature at which liquid changes to solid is known as :
(a) melting point
(b) boiling point
(c) freezing point
(d) condensation temperature
Click to show/hide
Q. 5 Which of the following options, is a mixture?
(a) Water
(b) Brass
(c) Iron
(d) Salt
Click to show/hide
