Rutherford’s Experiment and the Nuclear Atomic Model
Rutherford’s Experiment and the Nuclear Atomic Model :
In 1911, Ernest Rutherford bombarded a thin screen i.e. foil of gold (100 nanometer or 10-7 meter thick) encoated with zinc sulphide, with alpha particles (Helium nuclei). The following observations were made by this experiment :
- Most of the particles went out straight, without scattering.
- Some particles scattered at an angle of 90º and some at 120º angle.
- One out of 20,000 particles, however scattered at an angle of 180º i.e. returned on the same path after colliding with the foil.
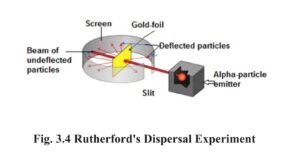
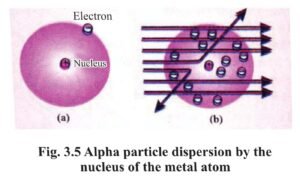
1. Rutherford deduced the following inferences from his experiment of dispersal of alpha particles on the gold sheet :
- Major part of an atom is a void.
- The entire positive charge of the atom is concentrated at a point.
- The space occupied by the positive charge is very less as compared to the volume of the atom.
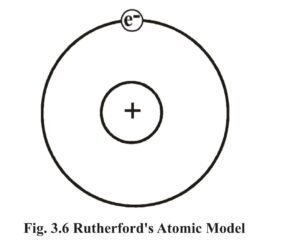
2. On the basis of these inferences Rutherford proposed the nuclear atomic model. The main points of this model are :
- The entire positive charge and mass of an atom is concentrated in a small part, the nucleus, at its center. The radius of the nucleus is 10-15 meter.
- Major part of the atom is void, in which the negatively charged electrons revolve at high speed on circular path, around the nucleus. These circular paths are known as orbit or shell or orbital.
- The ‘electrostatic force of attraction’ between the positively charged nucleus and the negatively charged electrons is balanced by the centrifugal force of the electrons revolving at high speed.
- The atom is electrically neutral because the total negative charge on electrons is equal to the total positive charge of the nucleus.
3. Drawback of the Rutherford’s Atomic Model.
- The negatively charged electron, revolving round the positively charged nucleus, will emit energy radiations because of acceleration; as a result, the energy will continuously decrease and ultimately the electron will fall into the nucleus. Hence the atom will not be stable.
- Rutherford could not explain the definite path for electrons.
Important Points :-
- The basic particles of atom are electron, proton and neutron.
- The negatively charged particles in the atom are electrons.
- The numeric value of the charge on electron and proton is the same but their sign is opposite.
- James Chadwick discovered neutrons.
- There are 6.022×1023 particles in one mole. This is known as the Avogadro number.
- The NTP volume of 1 mole of a gas is 22.4 litres.
- The formula to determine the maximum number of electrons in a shell is 2n2 .
- When the atomic number is the same but mass number is different they are known as Isotopes.
- Isobars are elements having different atomic number and the same mass number.
- There are three isotopes of hydrogen, Protium, Deutirium and Tritium.
Atomic Structure Important Questions-Answers
1. The Plum Pudding Model of atom was given by:
(a) Neil Bohr
(b) Thomson
(c) Ernest Rutherford
(d) Goldstein
Click to show/hide
2. The discoverer of neutron was :
(a) C.V. Raman
(b) Rutherford
(c) J.J. Thomson
(d) James Chadwick
Click to show/hide
3. The size of atom is :
(a) 10-6 cm
(b) 10-15 cm
(c) 10-2 cm
(d) 10-8 cm
Click to show/hide
4. The number of neutrons in the Deutirium Isotope of hydrogen is /are :
(a) one
(b) Two
(c) Three
(d) Not even one
Click to show/hide
